Following our last IP Alert of 23 January 2025, the decision of the European Commission dated 8 April 2025 (only the English text is authentic) is now available under reference AT.40588 : https://competition-cases.ec.europa.eu/cases/AT.40588
Facts
Teva commercializes Copaxone for the treatment of multiple sclerosis, notably in the European Economic Area (EEA).
Copaxone has long been Teva’s largest blockbuster. The active agent of Copaxone is glatiramer acetate (GA).
When its basic patent for GA approached expiry in 2015 and the competing Dutch manufacturer Synthon was preparing to enter the market with its GA product (Synthon GA), Teva devised an elaborate, multifaceted strategy to protect Copaxone from competition by prolonging its exclusivity and preventing or delaying the start of significant price competition from other GA medicines.
Between 24 and 28 October 2019, unannounced inspections pursuant to Article 20(4) of Regulation 1/2003 were carried out at Teva’s premises in the Netherlands, Germany and the United Kingdom. The inspections continued later on at the Commission’s premises and in the presence of Teva representatives. On 4 March 2021, the Commission opened proceedings with a view to adopting a decision pursuant to Chapter III of Regulation 1/2003.
Decision
1/ Market definition and dominance (see sections 6.3.4 to 7.3):
In each of the 7 investigated Member States (Belgium, Czechia, Germany, Italy, the Netherlands, Poland and Spain – the Relevant Member States or RMS), the relevant product market is the market for GA.
This is because the (approaching) market entry of Synthon GA has changed the nature of competition for Copaxone from non-price competition to price competition.
The Decision finds that Teva held a dominant position in each of the 7 RMS, in light of Teva’s very large market shares and significant net profits, high barriers to entry and expansion, lack of sufficient countervailing buyer power and Teva’s internal documents referring to its dominance and corroborating the Commission’s analysis.
2/ First abuse: Misuse of divisional patents (see sections 5.2.3.2 and 8 to 8.6):
The Decision concludes that Teva has committed an abuse of its dominant position in breach of Article 102 TFEU by misusing the patent system and patent procedures.
Teva’s conduct consisted of two intertwined practices :
a) First, Teva staggered filings of divisional patents in two patent families before the EPO that largely overlapped in content (see notably section 8.3). These patents shared essential features with ingrained legal weaknesses. They therefore shared the same risk of being revoked in validity challenges, which are a key instrument to remove unjustified patent barriers.
b) Second, Teva’s conduct consisted in the obstruction of effective legal review of these weaknesses through the strategic withdrawals of these patents before the EPO’s Technical Board of Appeals (see notably section 8.3.3). Thus no decision was adopted while one or more patents with similar overlapping claims were still in place.
Teva thus prevented the EPO from issuing a final decision on the validity of overlapping claims (i.e. no creation of any precedent case).
As a result of this combined conduct, generic companies had no option but to repeatedly restart their validity challenges against the remaining patents from scratch.
3/ Second abuse: Exclusionary disparagement (see sections 5.2.3.5 and 9 to 9.6):
The Decision concludes that Teva implemented a disparagement campaign against Synthon GA to hinder and/or delay its market entry and uptake in the RMS.
In particular, the Decision finds that:
(i) Teva disseminated objectively misleading information on key features of Synthon GA capable of discrediting it and which ran against the regulatory findings of the competent medicines agencies in the EU: specifically, Teva emphasized clinically irrelevant differences in molecular structures of Copaxone and Synthon GA; pointed to the risks observed in the use of other glatiramer-related substances, unjustifiably implying that they equally applied to Synthon GA; and called into question the scientific validity of the study on which the competent authorities based their finding of therapeutic equivalence between
Copaxone and Synthon GA;
(ii) Teva put in place effective mechanisms for the dissemination of the objectively misleading messages to relevant stakeholders (national authorities competent for pricing and reimbursement, health insurance funds and health care professionals) in the 7 RMS;
(iii) this conduct was capable of producing exclusionary effects; and
(iv) it was not objectively justified.
4/ Duration
The Decision finds that the first abuse (Teva’s misuse of divisional patents) started in all RMS on 3 February 2015, and ended at different dates depending on the RMS.
The Decision also finds that the second abuse (Teva’s disparagement campaign against Synthon GA) started in all RMS on 12 April 2016 (date of public announcement of approval of Synthon GA), and, according to a conservative approach of the Commission, ended at different dates depending on the RMS.
However, it is relevant to note that there is evidence of Teva referring to its disparaging messages on Synthon GA at least until 2021, and the company never implemented any corrective measures with respect to the dissemination of said disparaging messages.
Since these two abuses are complementary and aim to protect/strengthen Teva’s dominant position on the GA markets, they constitute a single and continuous infringement and last between 3 and 9 years depending on the RMS.
5/ Fines
The fine calculation is based on an average annual value of sales calculated over the entire period rather than the value of sales during the last full business year of the infringement. (relying on the revenue in the last full business year of the infringement would significantly understate the value of infringed sales and would not be representative of the economic importance of Teva’s single and continuous infringement).
The assessment of the gravity of the infringement takes into account the particularly serious nature of the infringement, the fact that Teva committed the infringement intentionally or at least negligently and an abusive conduct has been implemented, that Teva held high market shares in each RMS and that Teva’s infringement covered approximately 2/3 of Teva’s sales of Copaxone in the EEA.
The Decision considers that the two abusive practices were highly complementary and reinforced one another by being largely overlapping in time. Thus a higher gravity coefficient is applied for RMS where the two practices were both ongoing at the same time for more than 1/3 of the total duration of the single and continuous infringement.
Finally, for Czechia and Germany, a further increase to the gravity coefficient is applied, because of preliminary injunctions obtained on the basis of the divisional patents that were covered with Teva’s abusive conduct.
In order to contribute to the deterrent effect the fine should have on an undertaking of a size and with resources such as Teva, the Commission applied an additional amount of the relevant values of sales (= average value of sales of Copaxone x gravity coefficient used in the RMS).
No aggravating or mitigating circumstances were applied.
The Decision imposes a single fine of EUR 462 578 000 on Teva, which is calculated separately for each of the RMS:
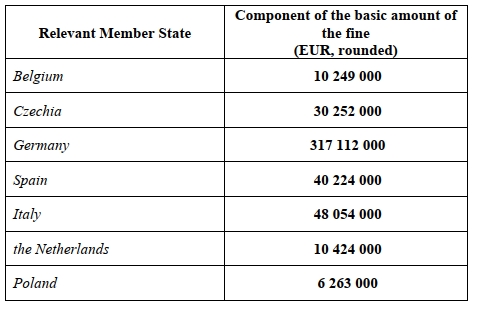
The calculated fines do not exceed 10% of Teva’s worldwide turnover.
This is the first time the Commission imposes a fine in relation to these two types of practices.